The innumerable players in the market offering an array of reliable, innovative options and rising awareness among consumers about the quality of end products have raised the bar so high that the nutraceutical industry must match consumer preferences to stay ahead of the competition.
To tailor-make products that match the cultural lifestyles of customers is one of the key factors responsible for driving the growth of today’s nutraceutical industry.
The current buzzword in the nutraceutical industry is “natural.” Consumers insist that, whenever possible, any additives used should be natural. Right now, there is no consensus regarding the meaning of the word … but it is accepted by most in the market that foods labelled as natural must be non-GMO (genetically modified organism) and should contain minimal or, if possible, no synthetic chemicals.
The increasing vegetarianism movement is the amalgamated effect of all these factors wherein consumers are now shifting from animal-based products to plant-based ones. Demand for clean label nutraceuticals is also growing tremendously among consumers, such that they want healthier products prepared using natural, plant based ingredients instead of artificial ones, and with less processing.
Cultural preferences and traditional dietary practices are other factors responsible for boosting the popularity of natural products; certain communities prefer products that are not prepared using any animal-based raw materials.
In such a scenario, HPMC (hydroxypropyl methylcellulose) capsules are being considered as the solution to many of the challenges mentioned above (Figure 1). The requirements for plant-based materials and ease-of-formulation properties to deliver a good quality product have favoured the use of HPMC capsules in the nutraceutical industry.
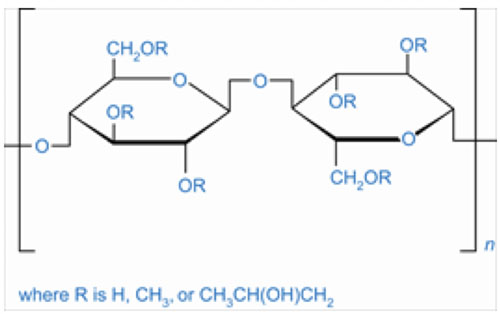
Figure 1: Hydroxypropyl methylcellulose (CAS 9004-65-3)
HPMC capsules: exemplifying vegetarianism
Patented in the 19th century, two-piece hard capsules have played a prominent role in the nutraceutical sector. Gelatin capsules were, for a long time, considered to be the market standard. In recent years, however, there has been a shift in thinking and, for many reasons, HPMC capsules have risen to the fore in the nutraceutical industry:
- the most important attribute of HPMC capsules is their vegetable origin: HPMC is produced from a plant resource — cellulose — which is obtained from either pine or poplar trees
- being plant-based, HPMC capsules offer strong product differentiation for vegetarian consumers
- they are non-GMO, Kosher, Halal, vegan-certified and approved by the Vegetarian Society
- HPMC capsules possess a lower moisture content compared with gelatin capsules and are therefore more suitable for moisture-sensitive formulations
- they are free from preservatives, allergens, starch and gluten
- permitted as a food additive for human consumption in accordance with 21 CFR 172.874 and regulation (EC) No. 1333/2008, HPMC is included in the US FDA inactive ingredients database and is recognised as a non-toxic and non-irritant material
- it is considered as GRAS (Generally Recognized as Safe) for use in food and has beneficial effects as a dietary fibre at levels up to 20 g/day.1–3
Why use HPMC capsules for nutraceuticals?
Moisture content plays a vital role in the stability of many nutraceutical products, including amino acids (acetyl-L-carnitine HCl, L-arginine base), vitamins (vitamin C, thiamine, vitamin B12, pantothenic acid), enzymes and coenzymes (NADH), certain minerals and their salt forms (copper, iron, zinc, magnesium chloride) and certain herbal preparations.4,5
For these formulations, in which moisture significantly affects the activity of the product, HPMC capsules offer an apt alternative owing to their low inherent moisture content. As well as having a low moisture content, HPMC capsules are completely resistant to cross-linking, thus make them compatible with a wide range of formulations.
For products containing probiotics, moisture presents a critical challenge. Probiotics are living micro-organisms, which, upon ingestion in adequate amounts, convey health benefits to the host. However, the conditions for the incorporation of viable probiotics need to be carefully considered.
The bacterial load can be lost or reduced during the manufacturing process, transportation and storage. There are several environmental factors that affect the viability of probiotics, such as titratable acidity, pH, dissolved oxygen, storage temperature, storage moisture content and the presence of other cultures.
During production, the probiotic is incorporated in a matrix to immobilise the micro-organism in a protective atmosphere and subsequently dried (using a process such as spray, fluidised bed or freeze drying). It has been determined that there is a critical storage moisture content value in which lactic acid bacteria can maintain their structural and biological function after drying.6
Figure 2 shows the viability of L. paracasei cells at 79% after 7 weeks of storage at 25 °C. The survival rate decreases as the moisture content increases. Observations indicate that there are no significative differences in viability in the range of moisture content from 2–6%.
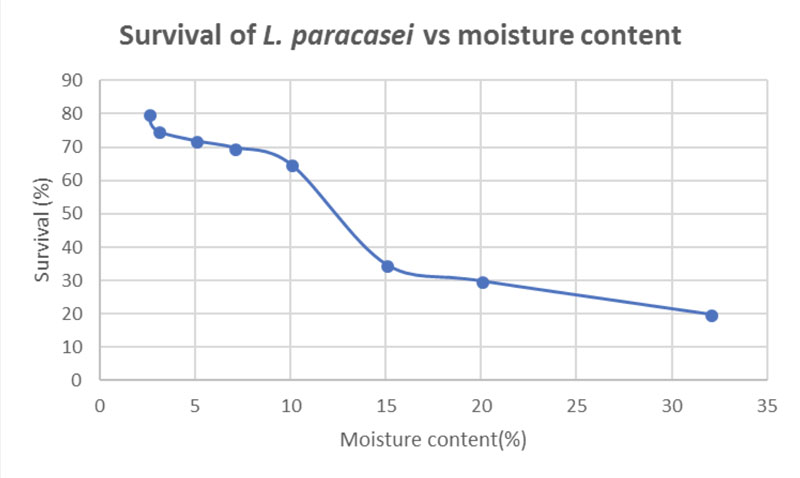
Figure 2: Survival of L. paracasei during 7 weeks of storage at 25 °C (adapted from reference 6)
Nevertheless, the survival of the micro-organisms was approximately 70% when the moisture content was 7–10%. This percentage of micro-organisms is equivalent to 8.9 x 108 CFU, which can be considered suitable for beneficial health effects.
Figure 3 shows the correlation between relative humidity (RH) and HPMC capsule moisture content. It can be seen that at an RH of 30–60% (normal RH conditions), the moisture content associated with HPMC capsules is 3–7%. This is the optimal value to encapsulate probiotics.
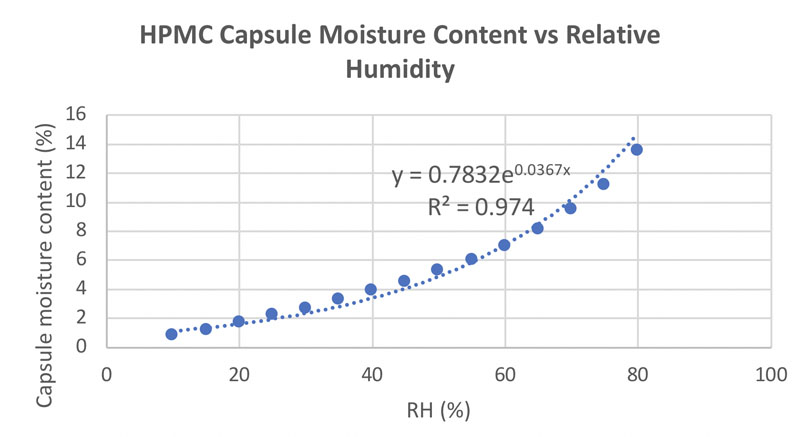
Figure 3: Correlation between moisture content and RH for HPMC capsules (adapted from reference 7)
There is an additional way to optimise the encapsulation-to-packaging process to maintain desired bacterial counts. After filling, HPMC capsules can be stored at a low RH.
Once a satisfactory equilibrium is obtained, the probiotic capsule can be blister packaged. This ensures a reduction in the moisture content that will be maintained at a low level.
If, for example, storage conditions are 20 °C and 11% RH, the final moisture content will be approximately 1%.7 There is no risk of the capsules becoming brittle at these low levels. Furthermore, unlike in gelatin, water does not act as a plasticiser in HPMC capsules. Special HPMC capsules with a moisture content of less than 3% are also available, which offer excellent packing and delivery properties.
Targeted delivery
As well as protection from moisture, HPMC capsules are excellent targeted delivery systems for gastric acid-sensitive nutraceuticals, including probiotics. The gastroresistant properties of HPMC capsules, owing to the presence of a suitable gelling agent, allows them to protect formulations that are susceptible to stomach acid degradation and deliver actives to absorption sites such as the intestine and colon.
Easy filling process
Capsules and tablets are the most commonly used dosage forms in the nutraceutical industry. Filling capsules is a relatively simple process, as the ingredients are introduced into the capsule at a reasonable output rate.
There are many encapsulation machines in the market that can complete the process in an efficient manner. Compared with encapsulation, tablet manufacturing is a somewhat challenging process because of additional processing steps such as compression and coating.
During compression, force is applied to the granules or powders for a specific period to form a compressed tablet. However, this exerts pressure on the formulation and the heat generated during compression can degrade some ingredients. Therefore, for certain nutraceutical products such as probiotics, tableting is not a recommended process.
As probiotics are sensitive to pressure, high compaction forces may damage the bacterial cells, which drastically decreases their viability (depending on the robustness of the strain).
Figure 4 shows the correlation between compaction force and the viability of the probiotic strain BB-12. Even at a moderate compression force of 10 kN, only 63% of the probiotic load survives the tableting process.
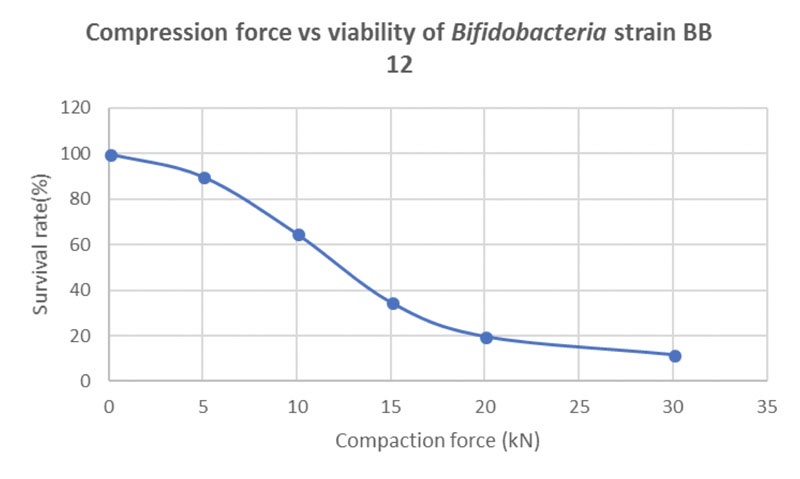
Figure 4: Correlation between compression force and bacteria cell viability in directly compressed probiotic tablets containing 20% of the bifidobacterial strain BB-12
It is therefore necessary to use special excipients if a tablet is the desired dosage form. Employing this strain in capsules would be the most appropriate and efficient route to ensure proper bacterial counts.
HPMC capsules display excellent mechanical strength and machinability, facilitating the filling process on any encapsulation machine. Besides, HPMC capsules are nearly identical in size to gelatin capsules and can, therefore, be filled using the same semi- or fully automatic high-speed encapsulation machines without modification. For probiotics, HPMC capsules offer a promising option ensuring both stability and easy processing.
References
- P. Dudeja, R.K. Gupta and A.S. Minhas (Eds.), Food safety in the 21st Century (Academic Press, Elsevier, Amsterdam, the Netherlands, 2017).
- S.B. Majee, et al., “HPMC as Capsule Shell Material: Physicochemical, Pharmaceutical and Biopharmaceutical Properties,” International Journal of Pharmacy and Pharmaceutical Sciences 9(10), 1–6 (2017).
- L.M. Tarantino, “Notification of the Determination of Hydroxypropyl Methylcellulose as Being Generally Recognized As Safe,” Keller and Heckman LLP (2006).
- www.naturalproductsinsider.com/manufacturing/solid-dose-nutraceuticals-pose-challenges?topic=fda.
- www.who.int/nutrition/publications/guide_food_fortification_micronutrients.pdf.
- M. Jiménez, et al., “Effect of Water Activity on the Stability of Lactobacillus paracasei Capsules,” Food Science and Technology 60, 346–351 (2015).
- www.ondrugdelivery.com/publications/80/ACG.pdf.
